Research Areas
For pressures and temperatures of the order of their critical-point values, fluids made of moderately complex molecules exhibit a thermodynamic behaviour which differs significantly from that of perfect gases. Flows of fluids in this particular regime are the focus of the branch of fluid mechanics called Non-Ideal Compressible Fluid Dynamics (NICFD).
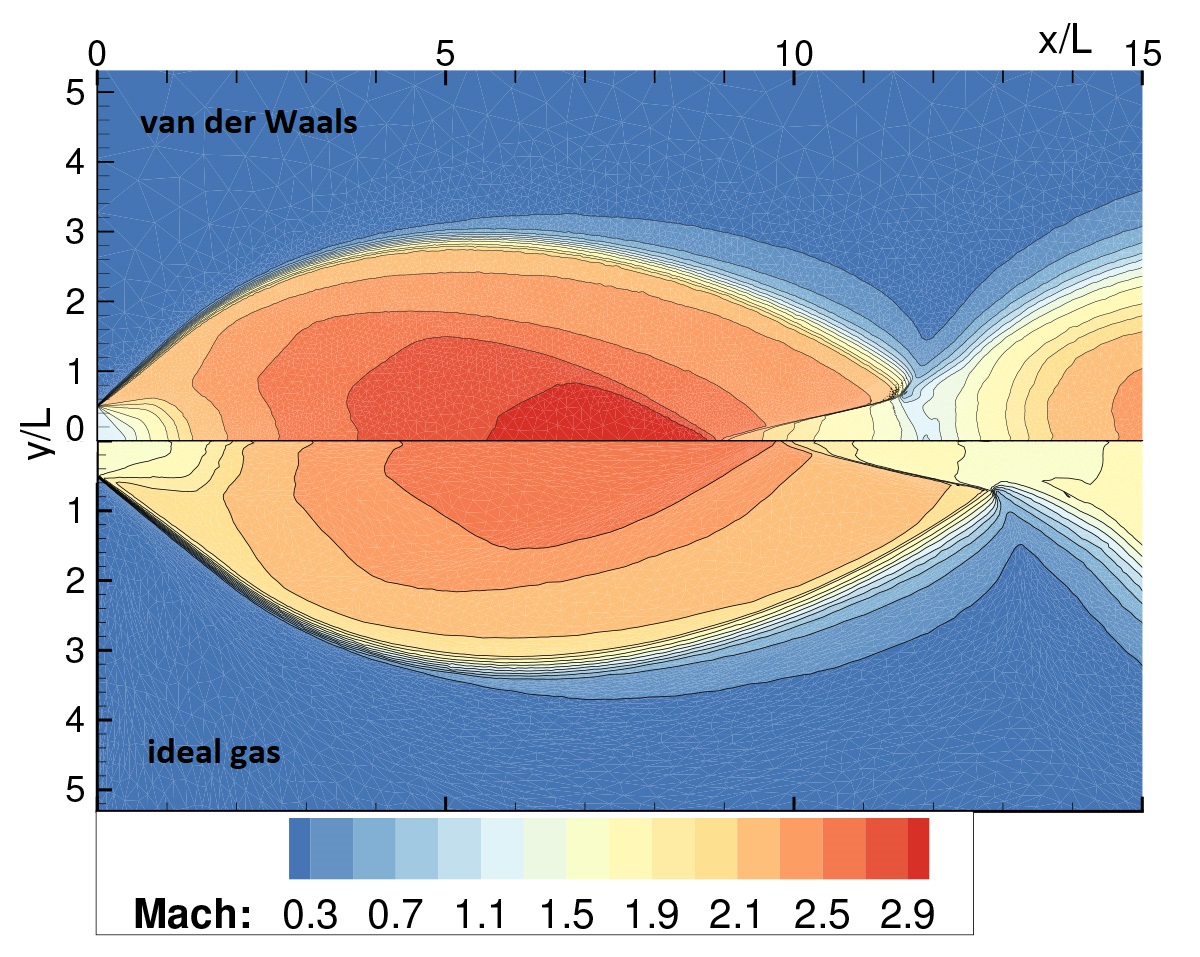
The departure from gas ideality, which is due to the interplay between attractive and repulsive intermolecular forces, is exemplified by the anomalous sensitivity of the speed of sound to density variations in isentropic processes. Relevant non-ideal effects include the non-monotonic trend of the Mach number along isentropic expansions and the Mach number increase across oblique shock waves (see figure). In addition, under special conditions, non-classical phenomena such as rarefaction shock waves and composite waves, can possibly occur. NICFD is relevant to various fields including ORC and supercritical CO2 power systems, refrigerators and high-temperature heat pumps.
NICFD is a core research area at the CREA Lab, where novel concepts and tools for the theoretical, numerical and experimental investigation of non-ideal compressible flows are constantly developed.
Experimental Methods
and Measurement Techniques
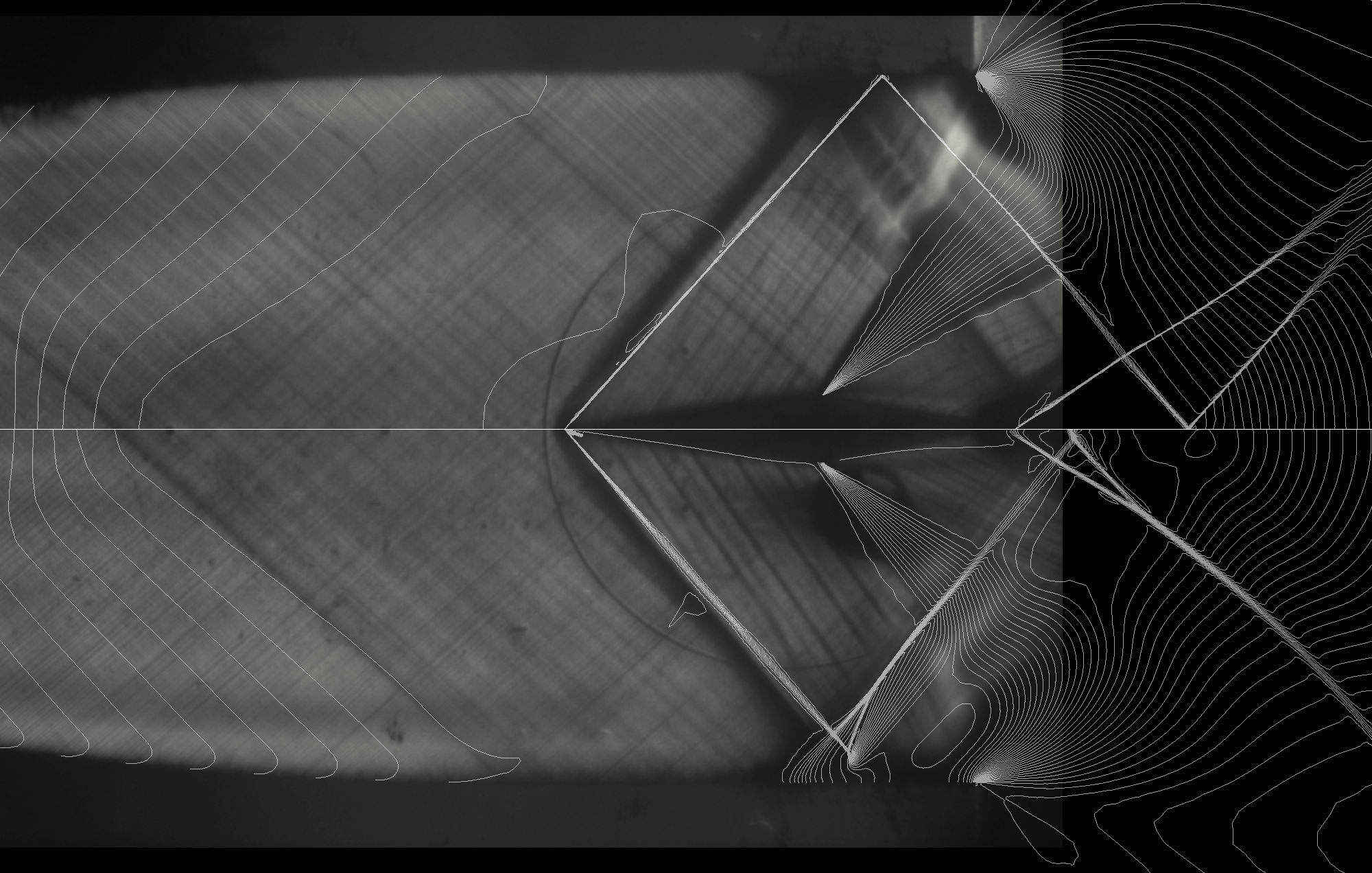
The experimental investigation in the field of gasdynamics has been historically conducted using wind tunnels. Studying non-ideal gasdynamics is however much more complicated since the fluids used are difficult to handle; they cannot be discharged in to the atmosphere, the test rigs must work at high temperature and they contain sections with sub-atmospheric pressures. The CREA Lab owns the TROVA facility that is so far, the only vapour tunnel running with organic vapour already in operation.
Experimental investigations on the TROVA facility are conducted together with several studies aimed at studying measurement techniques suitable for NICFD flows, such as Laser Doppler Velocimetry, Particle Image Velocimetry, and schlieren visualization.

Numerical Methods for NICFD
In conventional CFD solvers, the flow behavior is usually described through the polytropic ideal gas model. However, it is well known that fluids with complex molecular structure exhibit non-ideal gasdynamic behavior in a finite vapor-phase thermodynamic region near the saturation curve, e.g. in the so-called Non-Ideal Compressible Fluid-Dynamics (NICFD) regime. Consequently, the flow behavior departs from the ideal model and, under particular conditions, may even exhibit non-classical gas-dynamic phenomena.
Several research activities are under way at CREA Lab to develop state-of-the-art numerical techniques to accurately deal with non-ideal gas-dynamic behavior. In collaboration with the development team, the open-source CFD software SU2 has been equipped with a new built-in thermodynamic library encoding different equation of states and ad-hoc boundary conditions to model non-ideal flows. Similar capabilities have been implemented also in the in-house inviscid flow solver Flowmesh, in order to investigate mesh adaptation in non-ideal regime. Furthermore, additional research is devoted to develop more specific techniques in NICFD regimes, as for example, the method of characteristics for the design of the divergent portion of a nozzle and the computation of the existence domain of oblique shocks.
Non-Ideal Fluid Properties
The simplest description for thermodynamic properties of fluids is through the ideal gas equation of state. Though this formulation is accurate in some regions of the thermodynamic plane, the ideal gas law is not able to describe real fluid behaviour and does not make a distinction between different fluids.
The first equation of state capable of describing real fluid behaviour is introduced by Van der Waals. The van der Waals equation of state is capable of describing real fluid properties which depart from the ideal gas properties. Nowadays more complex equations of state are available to describe more accurately the thermodynamic properties of fluids and research is conducted continuously to improve these equations of state. These equations of state also introduce the ability to describe non-ideal gas dynamics behaviour of fluids with molecular complex structures e.g. siloxanes, fluorocarbons and perfluorocarbons.
Research activities and collaborations are ongoing at the CREA Lab to improve the description of thermodynamic properties of pure fluids as well as mixtures. Research has been conducted in collaboration with the National Institute for Standards and Technology, Boulder, United States, to develop state of the art equation of states for siloxanes mixtures. Furthermore, additional research is conducted to determine the thermal stability limits of siloxanes.
ORC and SCO2 Power Systems
Organic Rankine cycle (ORC) power systems represent a cost effective and mature technology for power production in the range 1 – 100 MW from thermal power available at low/medium temperature, as it is the case of renewables and heat recovery sources. In this context ORCs exhibit good efficiency and cycle configuration simpler than steam Rankine cycles. Supercritical CO2 (sCO2) power systems, represent an innovative technology which is gaining interest for the exploitation of clean sources and of nuclear energy in the medium/large power range. Among advantages of sCO2 cycles are the high conversion efficiency and plant compactness.
For both applications, turbomachines are key components and are operated with highly non-ideal compressible-fluids flows, occurring in the proximity of the vapor saturation curve and the critical point. Machine efficiency is expected to largely benefit from design tool refinement enabled by detailed studies on non-ideal compressible flows, with a significant impact on the overall conversion efficiency.
Thermal decomposition is a fundamental problem when dealing with organic compounds and can lead to a major loss of system efficiency.
At the CREA Laboratory typical non-ideal flows of interest for ORC and sCO2 application are studied by resorting to theoretical models and numerical simulations performed with SU2 suite. Also experiments are carried out in the TROVA facility for organic vapors flows, while the novel SCO2PRI facility for sCO2 applications is at the final stage of design. Further, with the THESTA facility it is possible to determine the thermal stability limit of organic fluids.

